Table of Contents
When water is blended with cement, the hydration process starts. The ingredients in concrete react with each other with the use of water and form more complex compounds. These compounds are called bogue compounds.
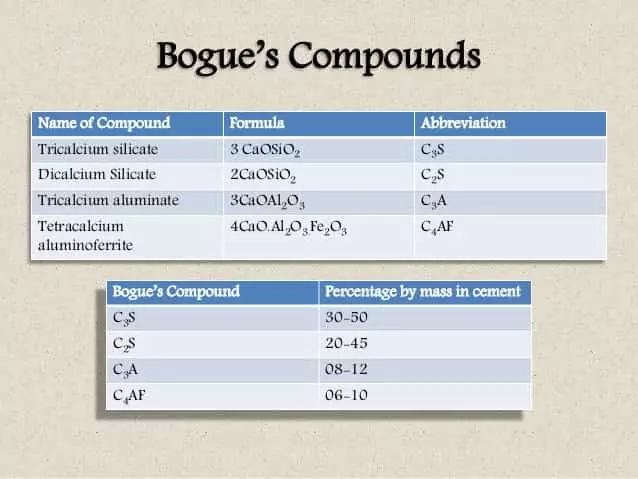
There are mainly four types of compounds that originated at the time of the hydration of cement.
Every compound possesses its features. The property of cement can be modified by changing the percentage of these compounds at the time of hydration.
There are numerous types of cement obtainable in the market according to the percentage of bogues compounds originated at the time of the hydration of cement.
The heat of hydration of bogues compound is about 8651/Cal. C4AF hydrates in enormous fractions premature a the time of setting while the final one to be hydrated are C2S. So, we can note the compounds in the declining ranking of hydration as C4AF, C3A, C3S, and C2S.
1. Types of Bogue Compounds
There are mainly four types of Bogue compounds that originated at the time of the hydration of cement.
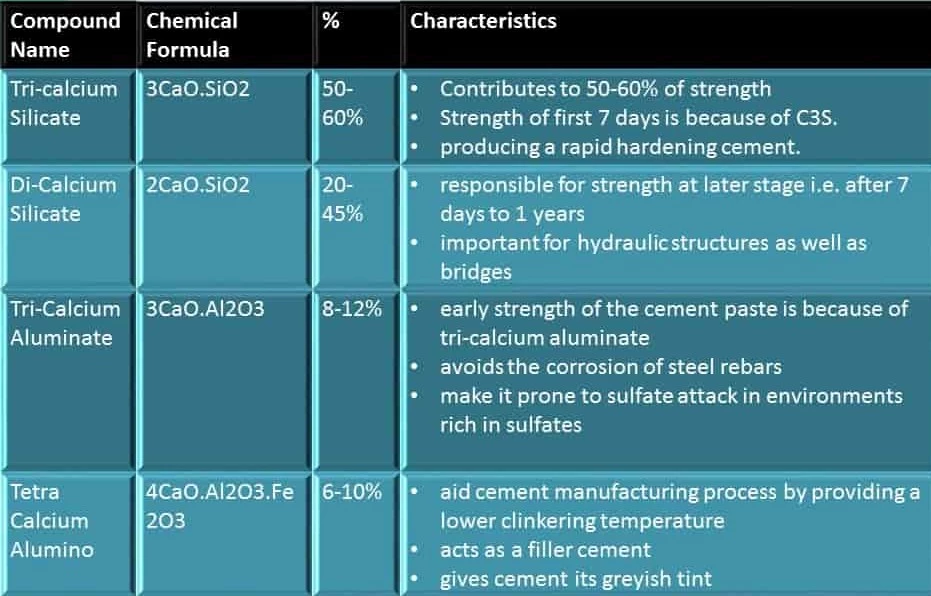
I. Tri-calcium Silicate (C3S)
The constituent that is available in significant percentage i.e. 30-50%.
It is chemically defined as 3CaO.Si02.
Around 50-60% of the cement paste strength is due to the existence and proportion of tri-calcium silicate. The strength of the first 7 days of the concrete flow is because of tri-calcium silicate.
Upon addition of water to the cement paste, the tri-calcium silicate act with water in an exothermic hydration reaction delivering calcium and hydroxide ions.
Once the process is saturated with ions, the calcium hydroxide forms crystallize thereby making calcium silicate hydrate at once.
These hydrate crystals get thicker thereby decreasing the rate of the reaction because of the problem for the water molecules to getting un-hydrated tri-calcium silicate.
In this way after crystallization, the hardened cement paste is produced. In concrete structures like pre-caste members or pre-fabricated construction, we want the concrete to be set fast and by raising the ratio of tri-calcium silicate we can accomplish this by making a rapid hardening cement. The case is identical to cold-weather concrete construction.
II. Di-calcium Silicate
Comparative to tri-calcium silicate, the di-calcium silicate is less reactive. It is also written as C2S and is chemically expressed as 2CaO.Si02. It is the second most large component of cement composite available about 20-45%.
The Di-calcium silicate acts with water, a slightly gradually, but an exact method of tri-calcium silicate. This is also an exothermic reaction but the heat made in it is way smaller than that of the earlier described reaction.
The di-calcium silicate, which is accountable for strength at a later stage i.e. after 7 days to 1 year, is very essential for hydraulic structures as well as bridges that require strength after years of service and detrimental effects.
Similarly, for constructions where smaller heat of hydration is required, like in low heat cement, the ratio of di-calcium silicate is modified consequently.
III. Tri-calcium Aluminate
It is available about 8-12% of the cement composite. Tr calcium aluminate is also written as C3A and is chemically expressed as 3CaO.Al203.
It is produced within 24 hours after mixing of water and the early strength of the cement paste is because of tri-calcium aluminate.
In areas where a quick set is required like in grouting of drain holes or to avoid water leakage the quantity of tri-calcium aluminate is raised.
But as it delivers a big quantity of heat of hydration, it must not be utilized much in the construction of mass concrete like in Roller Compacted Concrete hydraulic or water retaining structures.
The intense heat of hydration if stayed unattended will gives extreme cracking eroding the structural integrity and for this cause fly ash is kept to the mixture.
One of the very special advantages of its existence is that it bypasses the corrosion of steel rebars due to its chemical nature.
Tri-calcium aluminate merges with chloride ions to form CaCl2 thereby reducing the concentration of chloride accountable for the erosion of steel reinforcement. However, this part of C3A in cement type I and Ill are still being explored by scientists.
One of the most dangerous impacts of C3A on cement is to make it prone to sulfate attack in environments rich in sulfates. As the reaction of C3A with water is very quick that leads to an instantaneous solidifying of cement paste called flash set or false set.
To neglect this gypsum, (calcium sulfate) also termed Plaster of Paris, is kept in a ratio of 4-8 % during crushing of the cement clinker.
But this mixture of gypsum and C3A concurrently forms ettringite which later results monosulfate. This monosulfate then acts with sulfate in the external environment in an extremely expansive reaction causing an extreme material degradation.
IV. Tetra-Calcium Alumino Ferite
Tetra Calcium Alumino Ferrite is present as 6-10% in cement composite. It is also expressed as C4AF and is chemically expressed as 4Ca0.Al203.Fe203.
Tetra calcium alumino ferrite is not a compound that helps in the strength of cement. At the time of hydration reaction of cement, it results in a hydrated iron oxide gel in a quick reaction producing sufficient heat but the reaction delays down once calcium alumioferrite crystals are composed.
But it is kept to help the cement manufacturing process by supplying a lower clinker temperature. Besides this, it acts as a filler cement to make it cheap and also offers cement its greyish tint which is valued by the constructors.
2. Uses of Bogue Compounds
The uses of Bogue compounds are as follows:
✔ It hydrates & hardens gradually and delivers less heat of hydration.
✔ It supplies much of the ultimate strength & has more significant resistance to chemical attack.
✔ It delivers the highest heat of hydration & liable for the setting of cement.
| Read Also: Cement Price List |
